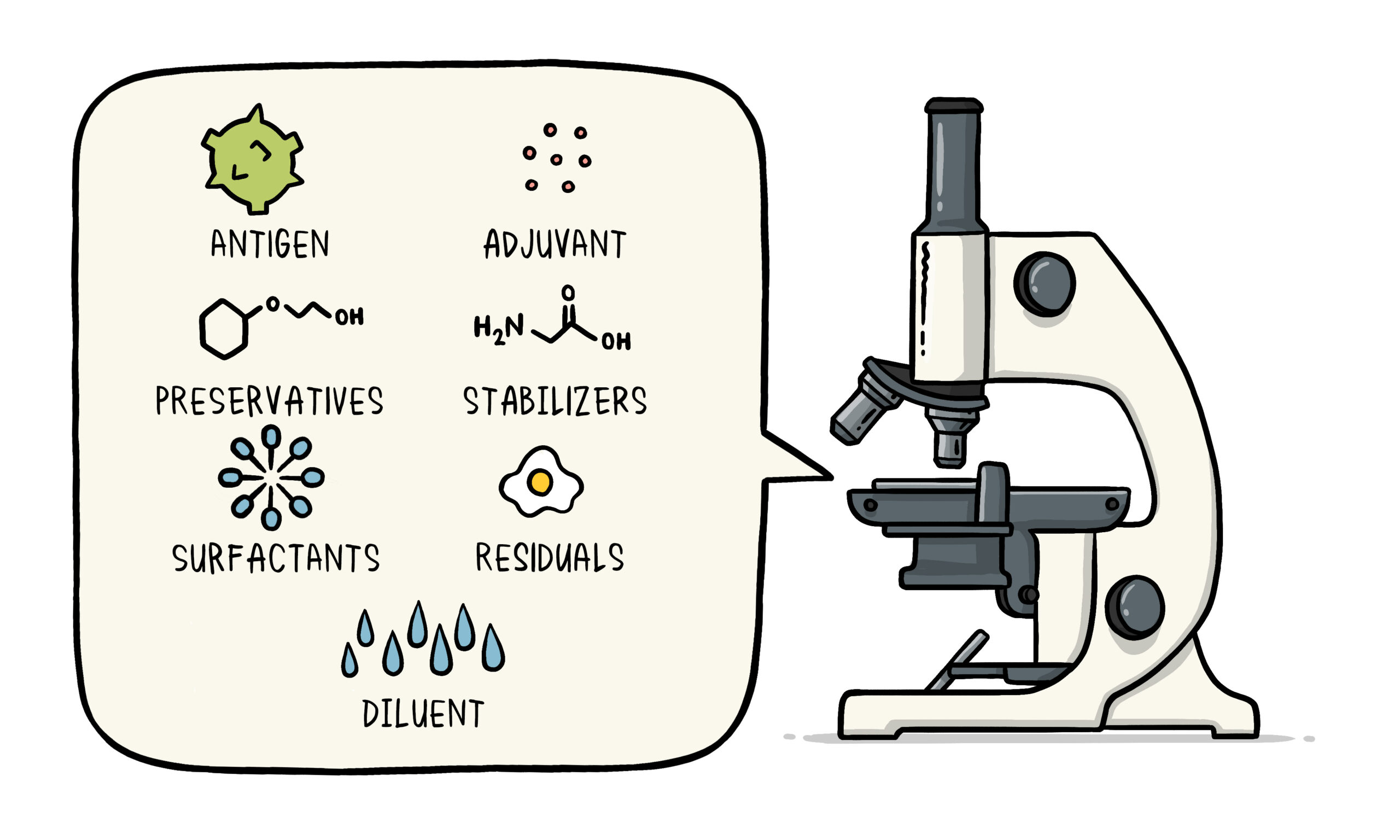
The Composition of a Chemical Cocktail
Image by the WHO, CC BY-SA 3.0 IGO, CC BY-SA 3.0 igo, https://commons.wikimedia.org/w/index.php?curid=98863670
WHO: What's in a Vaccine?
Vaccines are not pharmaceuticals—they are classified as "biologics" as traditional vaccines are derived from living organisms or their components. Modern vaccines include organisms formulated by scientists in a laboratory using biotechnology and genetic engineering.
This bio-chemical cocktail contains a variety of substances from antigens to excipients, added for a diversity of purposes, which are outlined below.
This is the key active ingredient intended to induce immunity against disease
It is what is considered the pathogenic agent, either bacterial or viral, either live-attenuated, inactivated or containing fragmented components (subunit). Since 2020, the novel mRNA gene-based platforms have been baptised "vaccines" with its antigenic material being the genetic code (to express a specific protein) wrapped in a nanolipid particle.
Below are a list of some examples of antigens in specific vaccines—different vaccines for the same disease can have different antigens based on the varying manufacturing processes adopted by vaccine producers.
Live-attenuated viral vaccines
- Measles:
The antigen in the measles vaccine is the live, attenuated (weakened) measles virus. This virus is most commonly derived from the Edmonston strain and is used in combination vaccines such as MMR (measles, mumps, and rubella). The antigenic components that stimulate the immune response are primarily the hemagglutinin (H) and fusion (F) proteins on the surface of the measles virus.
Most of the vaccines currently in use are derived from the Edmonston strain of the measles virus: Schwarz, Edmonston-Zagreb, AIK-C and Moraten. - Mumps:
The antigen in the mumps vaccine is the live, attenuated (weakened) mumps virus, most commonly the Jeryl Lynn strain (genotype A). This live virus is used in combination vaccines such as MMR and is grown in cell cultures (e.g., chick embryo or human diploid cells) and then attenuated so it cannot cause an active infection.
At the molecular level, the key viral proteins that are believed to elicit protective immunity are the fusion (F) glycoprotein and the hemagglutinin-neuraminidase (HN) protein on the virus surface.
- Rubella:
The antigen in the rubella vaccine is the live, attenuated (weakened) rubella virus, most commonly the RA 27/3 strain. This strain is grown in human diploid cells (WI-38) and is included in combination vaccines like the MMR and MMRV vaccines. - Chickenpox (varicella):
The antigen in the chickenpox vaccine is the live, attenuated (weakened) varicella-zoster virus, specifically derived from the Oka strain, grown on MRC-5 cell cultures. It is used in both the single vaccine (Varivax®) and the combination MMRV vaccine (ProQuad®).The original wild-type Oka strain (isolated from a child with chickenpox in Japan) is weakened by:
-
Serial passaging:
-
11 passages in human embryonic lung fibroblasts.
-
12 passages in guinea pig embryo fibroblasts (to reduce virulence for humans).
-
2–3 passages in WI-38 human diploid cells.
-
Final passages in MRC-5 human diploid cells (used for vaccine production).
-
-
Inactivated/toxoid bacterial vaccines
- Diphtheria:
The antigen in the diphtheria vaccine is the diphtheria toxoid, a modified, inactivated form of the diphtheria toxin produced by Corynebacterium diphtheriae. The toxin is rendered non-toxic (but still immunogenic) by treatment with formaldehyde. - Tetanus:
The antigen in the tetanus vaccine is the tetanus toxoid, an inactivated form of the tetanus toxin produced by Clostridium tetani. It is also detoxified using formaldehyde, maintaining its ability to induce an immune reaction.
Inactivated viral vaccines:
- Polio:
The antigen in the inactivated (IPV) polio vaccine is the killed (inactivated with formaldehyde) whole virus type for three strains of poliovirus Mahoney (Type 1), MEF-1 (Type 2), Saukett (Type 3) all grown on monkey kidney cells.
Inactivated recombinant subunit vaccines:
- Hepatitis B:
The antigen in the hepatitis B virus vaccine is the genetically engineered hepatitis B surface antigen (HBsAg). This is a purified protein found on the outer surface of the hepatitis B virus. In modern vaccines, HBsAg is produced using recombinant DNA technology: the gene for HBsAg is inserted into a plasmid and then introduced into yeast cells (Saccharomyces cerevisiae or Hansenula polymorpha), which then manufacture, or "express" the protein. The protein is harvested, purified, and used as the active ingredient (antigen) in the vaccine.
Some newer formulations, such as PreHevbrio®, include three versions of the surface antigen: the small (S), middle (pre-S2), and large (pre-S1) hepatitis B surface proteins. However, most widely used vaccines in Western nations (like Engerix-B® and Recombivax HB®) contain only the S protein.
- Pertussis (whooping cough) – acellular
The pertussis vaccines used in most high-income countries contain purified, inactivated components (antigens) of the Bordetella pertussis bacterium, the main antigens being:-
Pertussis toxoid (PT): a detoxified form of the pertussis toxin
-
Filamentous hemagglutinin (FHA): an adhesion protein that helps the bacteria attach to respiratory tract cells
-
Pertactin (PRN): another adhesion protein
-
Fimbriae types 2 and 3 (FIM2/3): surface proteins involved in bacterial attachment
-
Nucleic-acid based:
- Covid-19: mRNA coding for coronavirus spike protein S1
The gene-based antigen is a protein encoded by the mRNA—commonly the spike (S) protein of SARS-CoV-2 for COVID-19 inoculations. The mRNA instructs cells to produce this protein, which is intended to express itself on the surface of the cell to trigger an immune response resulting in host immunity against the disease.
Influenza (inactivated and live-attenuated)
The antigens for influenza vaccines are the viral strains of influenza type A and type B. These vaccines that can be inactivated or live-attenuated, oral/nasal-spray or injected, and are either cell- or egg-based. Typically 2-3 subtypes of influenza strain type A (for instance H1N1** subtypes: A/Hawaii/70/2019, A/California/7/2009 (H1N1)pdm09-like, A/Wisconsin/67/2022, A/Victoria/4897/2022 or H3N2 subtypes), and one lineage of type B strains (Victoria, Yamagata) are included in annual flu vaccines as antigens.
The strains included in annual flu shots are decided by an advisory committee at the WHO who meet, twice a year, to identify what viral strains are likely to circulate the following winter, and to make recommendations on the flu vaccine antigens. These recommendations are announced in February for the Northern Hemisphere and in September for the Southern Hemisphere.
**"H1N1" refers to a group defined by specific surface proteins: hemagglutinin type 1 (H1) and neuraminidase type 1 (N1). H1N1 describes the category of viruses, while vaccine strains are selected representatives from within that subtype.
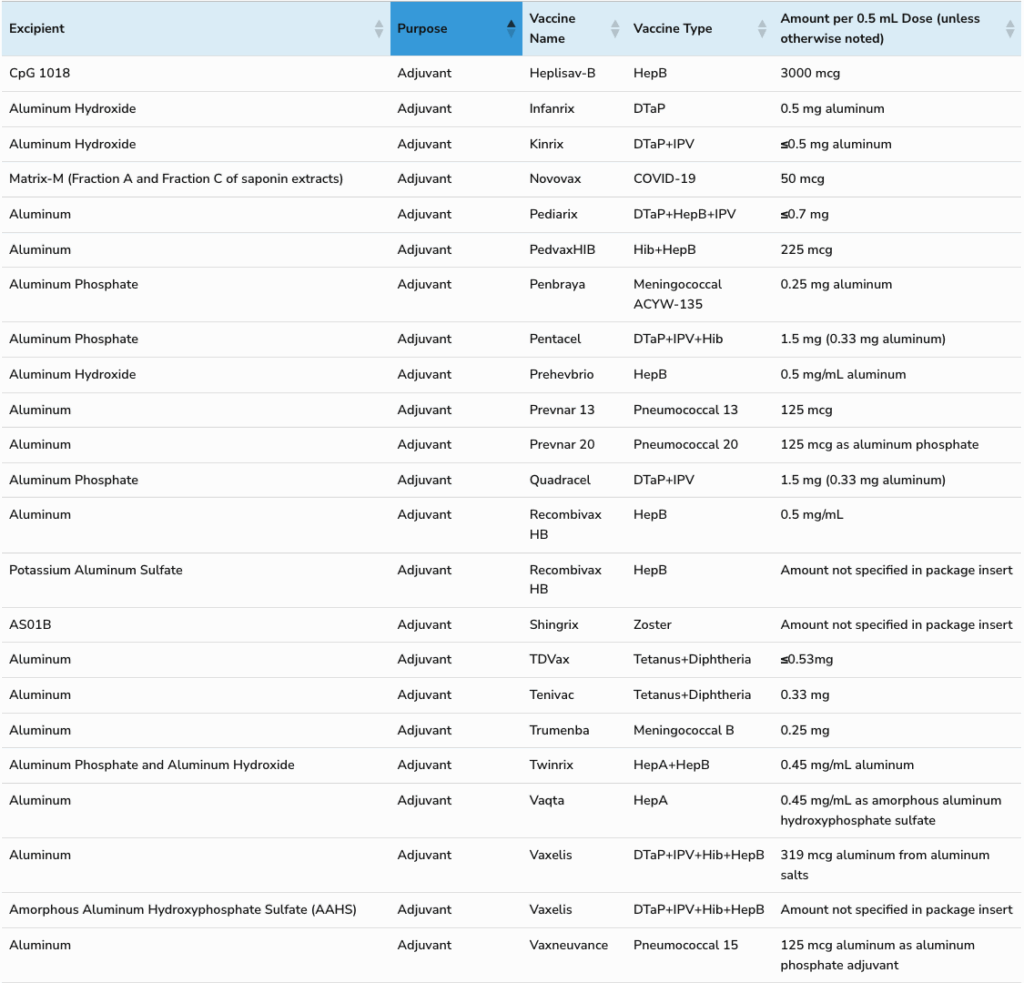
Aluminum Adjuvants
Aluminum adjuvants have been used from the early 20th century, first included in diphtheria and tetanus toxoid vaccines.
Aluminum hydroxide and aluminum phosphate are the most popular vaccine adjuvants in use today. The 21st century welcomed the arrival of Merck's proprietary nanosized amorphous aluminum hydroxyphosphate sulfate (AAHS), included in the following vaccines:
-
Gardasil (HPV vaccine for types 6, 11, 16, 18)
-
Gardasil 9 (HPV vaccine covering additional types)
-
Recombivax HB (Hepatitis B vaccine)
-
Vaqta (Hepatitis A vaccine)
-
Vaxelis (DTaP-IPV-Hib-HepB combination vaccine)
-
Procomvax (Combination vaccine for Hepatitis B and Haemophilus influenzae type b)
AAHS is used specifically in these formulations to enhance the immune response to the vaccine antigens.
Read below two interesting articles that address the official exposure guidelines and study the cumulative exposure of aluminum via injected vaccines recommended on the CDC schedule.
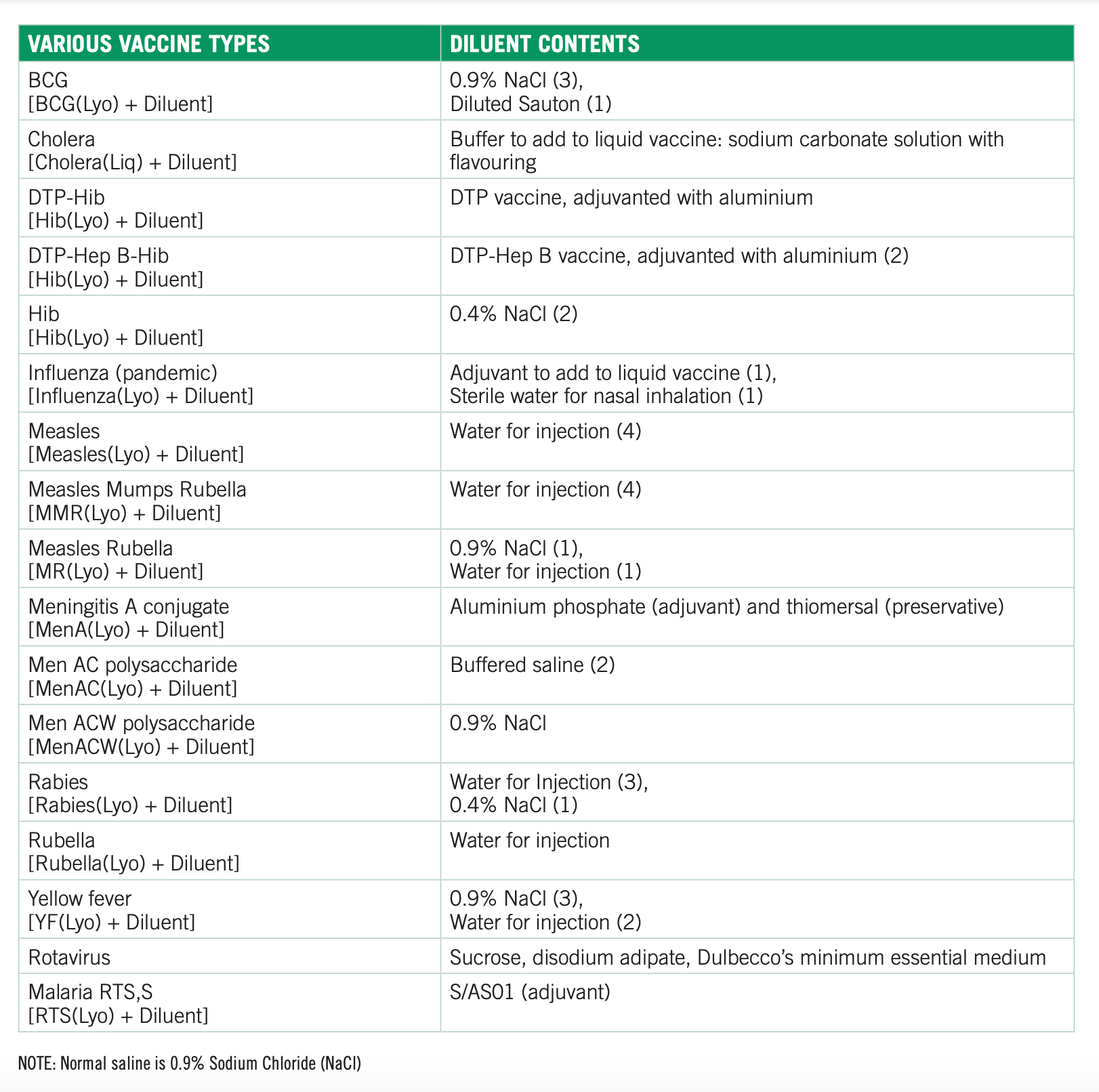
Diluents
Many vaccines are supplied freeze-dried and need to be reconstituted with its diluent before injection.
Diluents include sterile water, saline solution, or in the case of malaria RTS,S/AS01, the AS01 adjuvant suspension. The diluent of the MenA(Lyo) meningitis vaccine contains both an aluminum adjuvant and thimerosal preservative, and is given to children in Africa from 9 months old.
Detailed Lists of Ingredients
Johns Hopkins' Institute for Vaccine Safety (USA)
The Johns Hopkins Institute for Vaccine Safety (IVS) was founded in 1997 at the Johns Hopkins University School of Public Health, established by Dr. Neal A. Halsey, who served as its founding director. Its purpose is to provide independent assessments of vaccine safety to guide decision makers and educate physicians, the public, and the media about vaccine safety issues.
IVS provides one of the most detailed and easy to search list of ingredients for vaccines available on the US market through its Institute for Vaccine Safety website.
University of Oxford – Vaccine Knowledge (UK)
The Vaccine Knowledge Project was set-up in 2011 (rebranded in 2023) as part of the Oxford Group, which is part of the Department of Paediatrics at the University of Oxford, under the leadership of Sir Andrew Pollard. Its website provides links to all Summary of Product Characteristics (SPC) and Patient Information Leaflets (PIL) for vaccines available in the UK.
"Since July 2016, the Vaccine Knowledge Project has been a member of the WHO's Vaccine Safety Net, a global network of websites providing reliable information on vaccine safety. This means that this website has been judged to meet the WHO's criteria for providing good quality information about vaccine safety issues."
Children's Hospital of Philadelphia (CHOP) – Vaccine Education Center (US)
The Vaccine Education Center (VEC) was launched in October 2000 to provide "accurate, comprehensive and up-to-date information about vaccines and the diseases they prevent."
The Center was founded by Dr. Paul A. Offit, pediatrician, vaccinologist, and professor of pediatrics at CHOP and the University of Pennsylvania, who continues to serve as its director.
CHOP's Vaccine Education Center is a member of the WHO's Vaccine Safety Net, a global network of websites providing reliable information on vaccine safety.
Generally Recognized as Safe (GRAS) and Trace Amounts
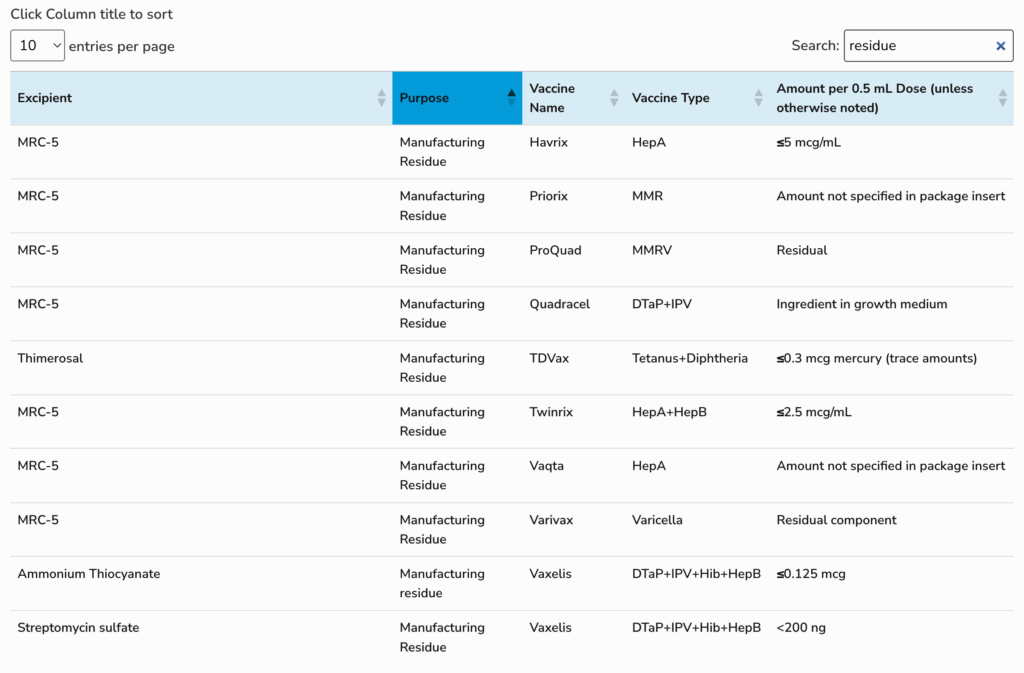
While vaccine manufacturers are legally obligated to disclose most ingredients, there are some tiny or "trace amounts" of substances that are not listed—sometimes to protect trade secrets of excipients "Generally Recognized As Safe" or GRAS.
Trace amounts are generally considered ingredients that are less than 0.5mcg/μg micrograms per mL, or less than 500ppb parts per billion.
While not an official ingredient, traces of the pesticide glyphosate have been detected in vaccines, with the highest amounts measured (2.671ppb) in the MMR vaccine.
Excipients which originated in the food industry (bovine serum, bovine albumin, gelatin) and others (Tween 20, sorbitol, tris, phenol, Ethylenediaminetetraacetic acid (EDTA), 2-phenoxyethanol) typically have a GRAS status by the US FDA (see Table 1).
Vaccine ingredients lists and product information on vaccines available in other countries than the US
Important Books
Epidemiology and Prevention of Vaccine-Preventable Diseases "The Pink Book", 14th Edition (2021)
The Pink Book, provides physicians, nurses, nurse practitioners, physician assistants, pharmacists, and other healthcare professionals with the most comprehensive information on routinely used vaccines in the USA—immunization recommendations, vaccine safety, storage, handling, administration, and the diseases these vaccines are supposed to prevent.
The Vaccine Handbook: A Practical Guide for Clinicians – 12th Edition (2024)
"The Purple Book, by Gary S. Marshall, MD, is an authoritative, user-friendly guide to almost everything related to immunization. Easy to navigate yet replete with up-to-date information, this handy resource contains practical advice and background on vaccine program infrastructure, standards and regulations, business aspects of vaccine practice, general recommendations, schedules, special circumstances, and how to address the concerns. Specific information about vaccine-preventable diseases, the rationale for vaccine use, and available products is included."
Plotkin's Vaccines – 8th Edition (2023)
"From the latest vaccination evidence, recommendations, and protocols . . . to new vaccine development and the use of vaccines in reducing disease, Plotkin’s Vaccines, 8th Edition, covers every aspect of vaccination. Now completely revised and updated from cover to cover, this award-winning text continues to provide reliable information from global authorities, offering a complete understanding of each disease, as well as the latest knowledge of both existing vaccines and those currently in research and development. Described by Bill Gates as "an indispensable guide to the enhancement of the well-being of our world," Plotkin’s Vaccines is a must-have reference for current, authoritative information in this fast-moving field."
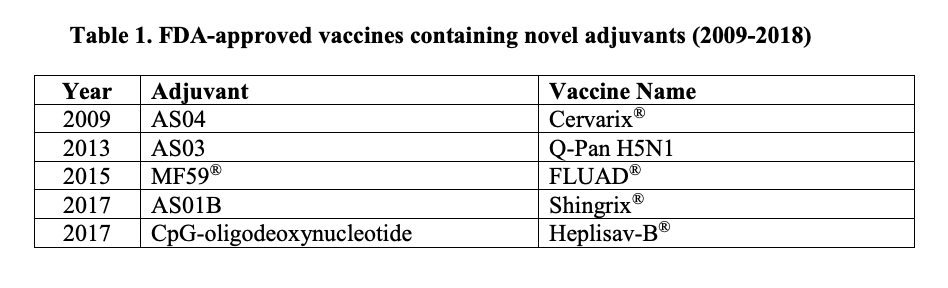
NIAID Strategic Plan for Research on Vaccine Adjuvants (2018)
"The use of adjuvants began in 1920 when the French veterinarian Gaston Ramon discovered that co-administration of inactivated diphtheria toxoid with starch, breadcrumbs, or other substances led to an increase in antitoxin responses to diphtheria. In 1926, this finding was followed by Alexander Glenny’s discovery of the adjuvanticity of aluminum salts and their clinical utility in diphtheria immunization.
Until 2009, aluminum-based adjuvants, collectively termed “alum,” were the only adjuvants in vaccines licensed for use in the United States. That year, the U.S. Food and Drug Administration (FDA) licensed the first of five vaccines containing novel [non-alum] adjuvants, which are described and summarized in Table 1 [see adjuvant tab above]."
BioPlan Associates – Biopharmaceutical Products in the US and European Markets (2007)
This two-volume reference book is a treasure trove of historical, scientific and commercial information on vaccines. From vaccine approval dates to US vaccine annual sales revenues and from vaccine ingredients to patent disputes, this publication is the "king of biopharmaceutical reference books". As reviewer Gordon Kelley put it, "describing [this book] as 'comprehensive' is a bit like defining botulinum toxins as merely pathogenic."
No Placebo Necessary
No Placebos in Childhood Vaccine Pre-licensure Clinical Trials
The public places its trust in the government regulatory agencies tasked with protecting the population from dangerous drugs and devices. These agencies, like the FDA, Health Canada, MHRA, EMA, Swissmedic, TGA etc., put their trust in the pharmaceutical industry, who alone provide the scientific data that supports the documentation submitted for the approval process. Too often, the regulatory agency is dependent on the vaccine manufacturers, also called "sponsors", for the financial health and survival of its organization, which creates an implicit conflict of interest.
Most of the ingredients included in vaccines are assumed to be safe due to "historical use" and often do not have to individually tested for toxicity. No vaccine is studied for its potential to cause cancer or to mutate genes and only some vaccines are tested for reproductive toxicity in a limited number (less than 100) of mice.
The parents of any country would want the injected chemical cocktail given to their baby to have been tested in a randomized placebo-controlled double-blinded clinical trial, over at least two years, to establish an authentic safety profile for the vaccine, no?
New York attorney-at-law, Aaron Siri Esq., has made available a summary report on the lack of true placebos used for vaccine pre-licensure clinical trials. Take note that the very definition of "placebo" has been manipulated to mean other vaccines, excipients and/or aluminum adjuvant.
Useful Websites on Vaccine Ingredients
Though currently defunct, the WAVE website still contains valuable information accessible via the wonderful Internet Archive Wayback Machine.